
Compliant & efficient SAE/SUSAR collection, processing, and reporting with automatic archiving and real-time analysis




One-click follow-up report creation with over 80% accuracy
Reuse over 40 fields without re-entry, improving accuracy
Lab monitoring forms are auto-imported with intelligent recognition, no manual input needed
OCR automatically scans and imports paper records, eliminating manual entry
Automatic duplication detection and discrepancy checks improve efficiency and reduce errors
Reports are received online and automatically generate SAE processing tasks
Automatically capture documents from the entire process: collection, processing, submission, and distribution
Automatically generate corresponding folders and directory structures
Integrated with Taimei Technology’s eTMF, eliminating the need for manual archiving
Served over 500 domestic and international clients
Comprehensive after-sales service with quick response to regulatory changes
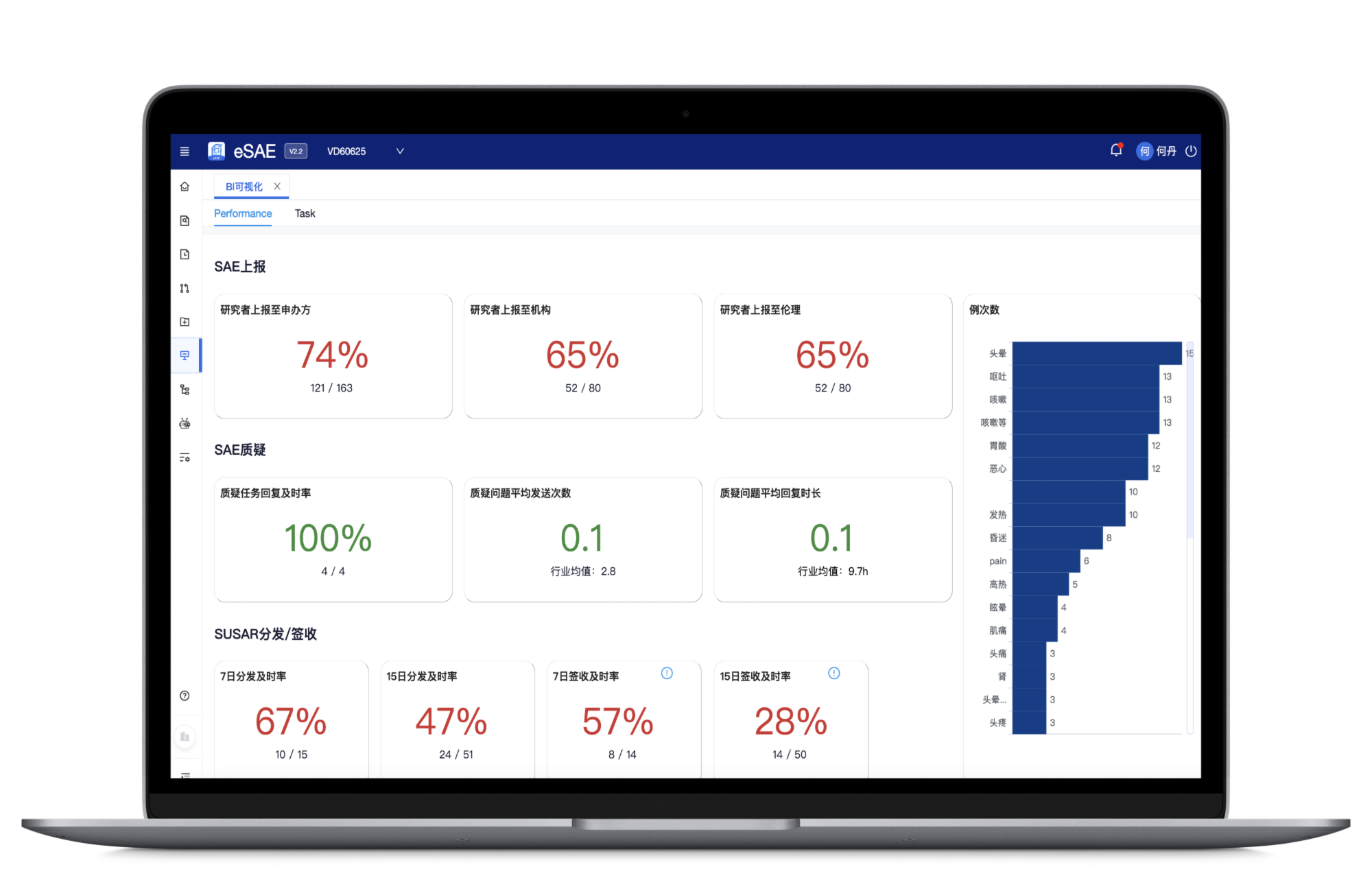
Display task progress, safety trends, and key business metrics to help PMs/CRAs track SAE progress and risks, supporting decision-making
Countdown reminders for timely report processing and submission
Stay updated with industry trends and regulatory interpretations

Scan to Follow Us on LinkedIn

Scan to Follow Us on WeChat