
Institution-Centered Platform for Comprehensive, Standardized Clinical Research Management
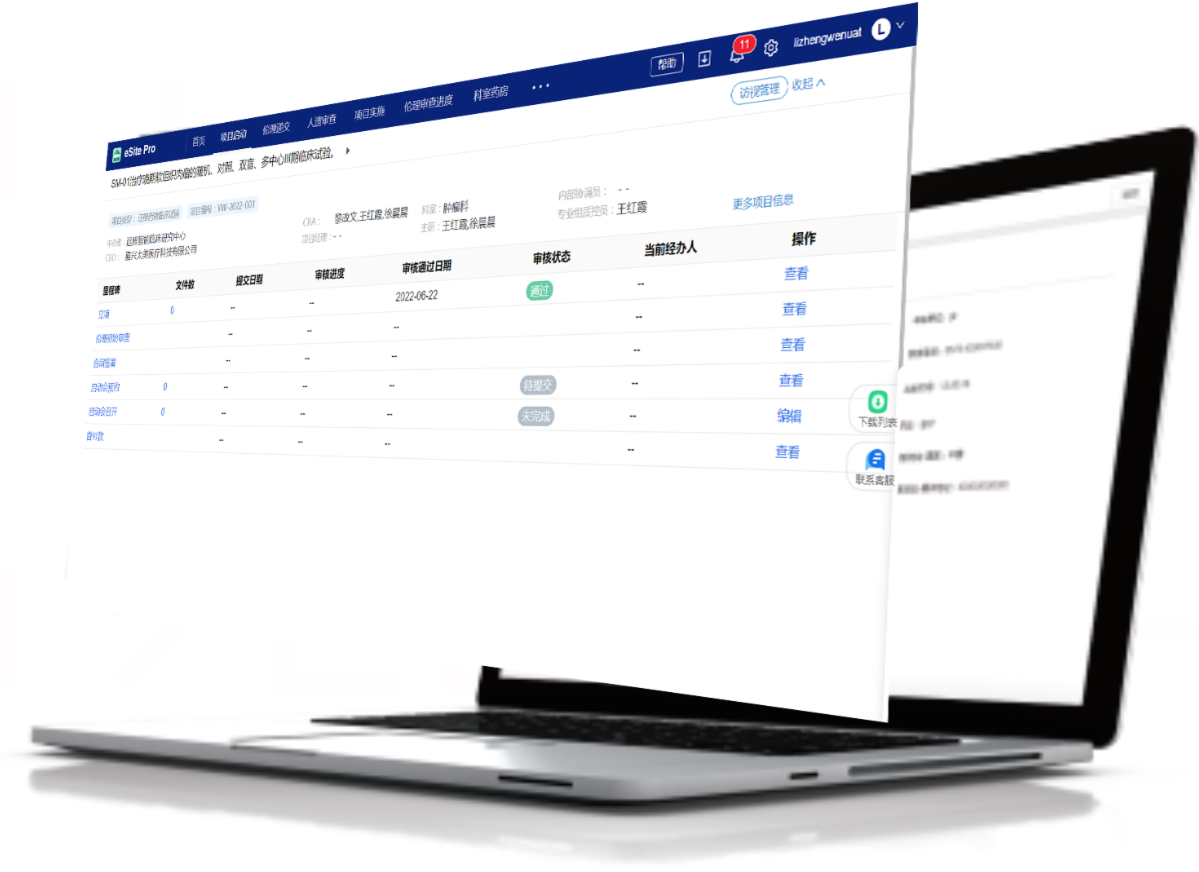
Covers the full project lifecycle—from initiation and agreements to implementation and close-out, ensuring progress control
Customizable approval processes to meet site requirements with real-time alerts to boost efficiency
Accurate financial tracking and breakdowns for effective trial expense management
Visual reports on project progress and distribution, with customizable options

Efficient online collaboration with fully automated ethics review processes to enhance efficiency
Customizable forms with one-click export to reduce workload
Compliant with both research ethics and GCP standards
Prepare ethics meetings in under 30 minutes, with online voting and automatic tallying. One-click generation of approval documents
Track review decisions and status in real time
Free testing and examination plans to improve subject compliance
Independent GCP-compliant billing bypasses patient payments and insurance claims, ensuring compliance
Follow-up packages based on trial protocols with GCP-compliant medical orders to reduce protocol deviations
Integration with HIS and financial systems for automatic billing, project accounting, and performance tracking

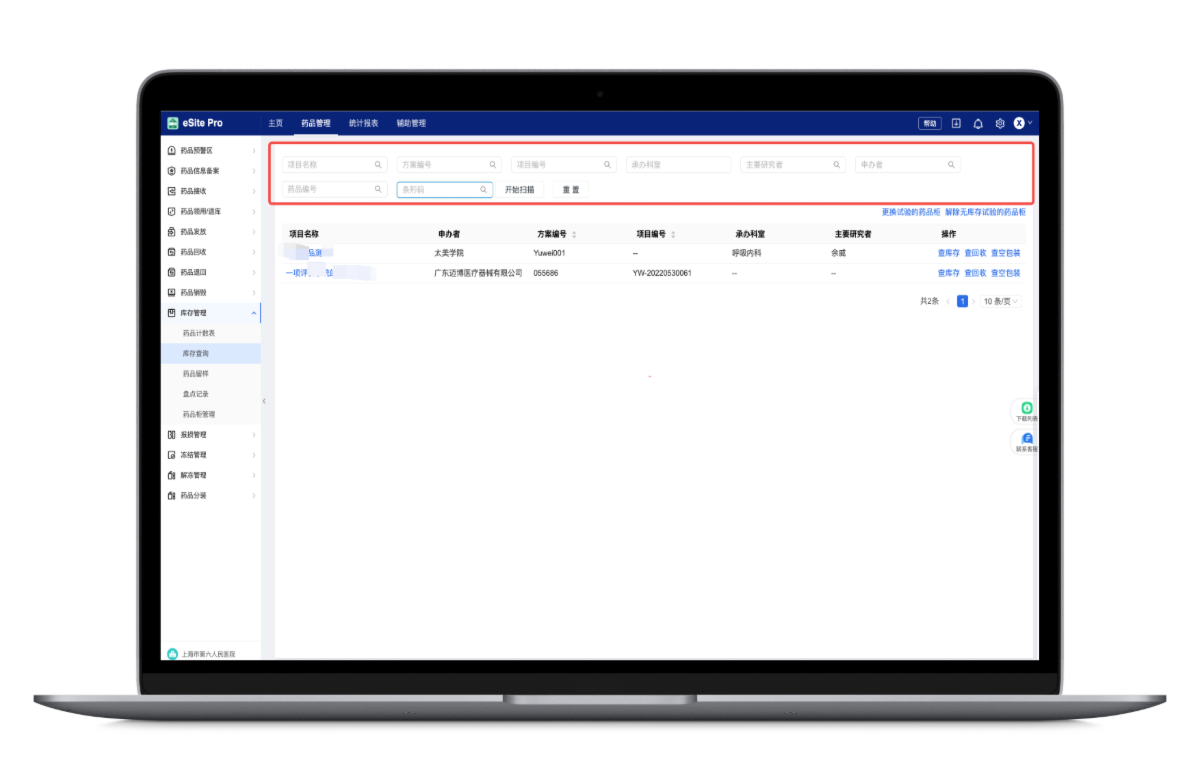
Investigational Drug Services (IDS): Automatic counting and logic verification ensure zero errors
Supports detailed management of various drug packaging for complex projects
Prescription and drug verification via barcode scanning guarantees error-free dispensing
Automated validation of drug expiration, dosage, and empty packaging recovery ensures data accuracy
Drug-related forms and data are automatically generated and compiled, significantly reducing workload for researchers and drug managers

Supports various research project applications with scientific review committees to ensure rational project planning
Detailed management of research funds to ensure proper usage
Manage project progress, mid-term reviews, and changes to ensure smooth execution
Consolidate research achievements across the institution with linked projects for easy, dimension-based statistics
Quantify and assess research outcomes, making it easier for managers to track rewards and boost research motivation

Scan to Follow Us on LinkedIn
