
End-to-End Clinical Drug Management with Precision and Safety

Barcode label management for drugs and reports
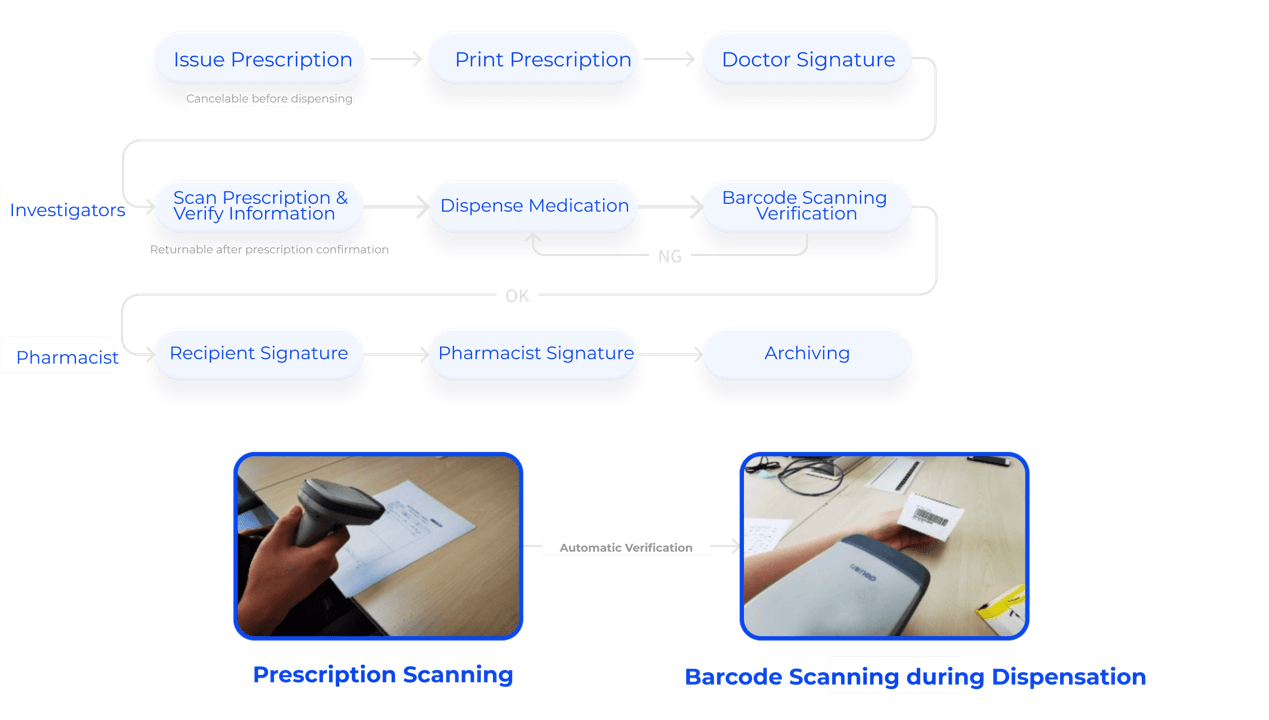
Barcode scanning verification for drug dispensing and returns
Dual pharmacist verification
Automatic validation & control of drug expiration dates, quantities, and empty packaging recovery
Automatic counting and trace recording throughout the entire process


Prescriptions and drugs automatically verified
Automatic validation of drug expiration, usage quantities, and empty packaging recovery

Auto-generated forms and data aggregation, greatly reducing workload for researchers and drug managers
Convenient data queries enable quick service response

Rapid audit and verification response
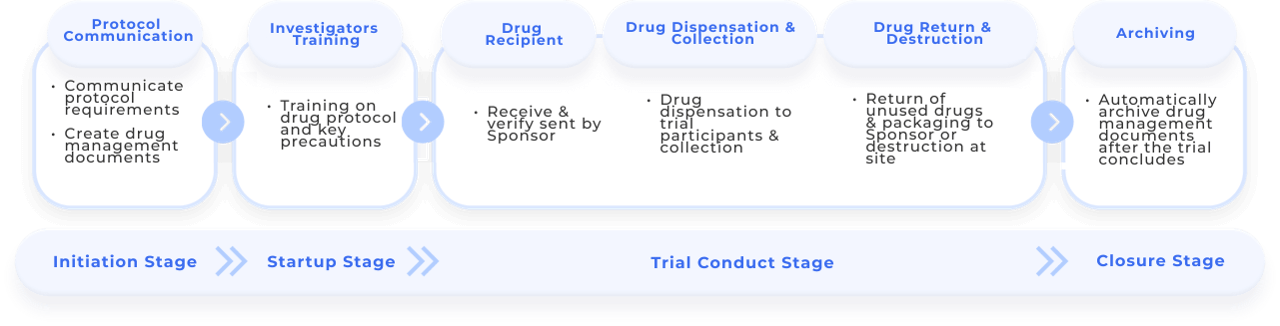
More time for protocol training, and communication during drug administration

Drug process information automatically locked
Automatic log tracking

Scan to Follow Us on LinkedIn

Scan to Follow Us on WeChat