
Smart Data Platform desiged for Trial Institutions for Seamless Clinical Trials and Accurate Data Support
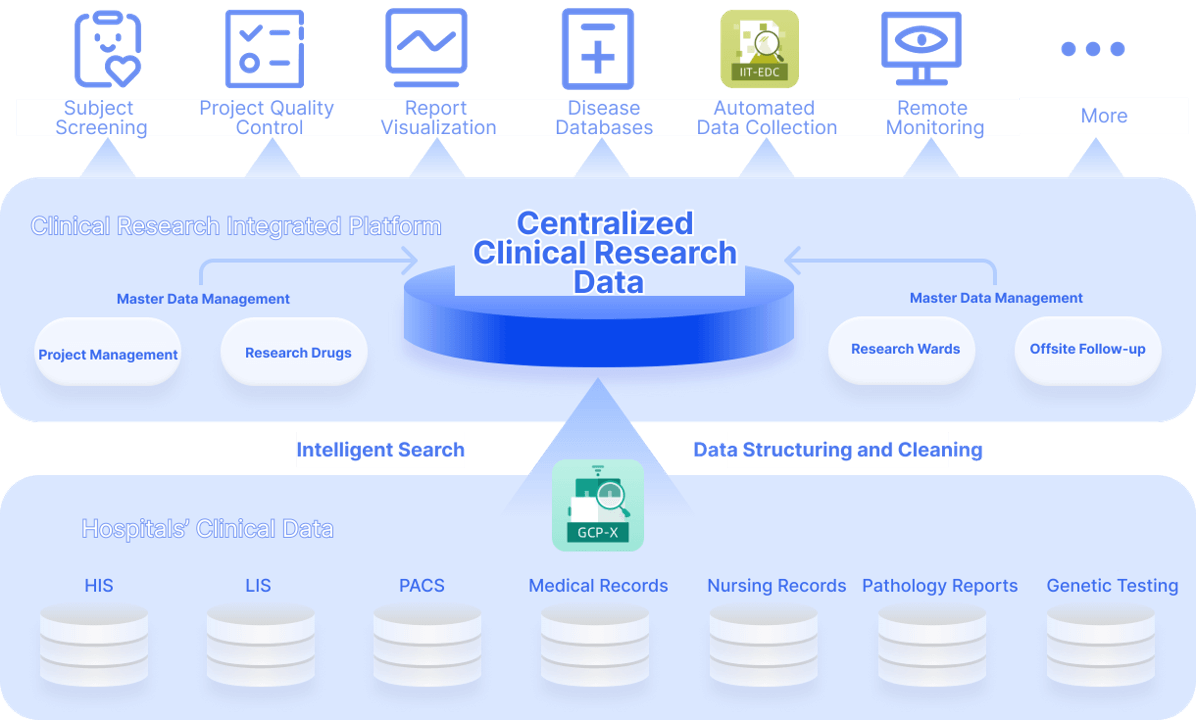
Consolidates clinical data from HIS, LIS, EMR, PACS, medical records, medication logs, nursing records, pathology reports, genetic tests, and more. After data cleaning and standardization, it provides a panoramic view of all original data from the patient’s clinical trial journey.
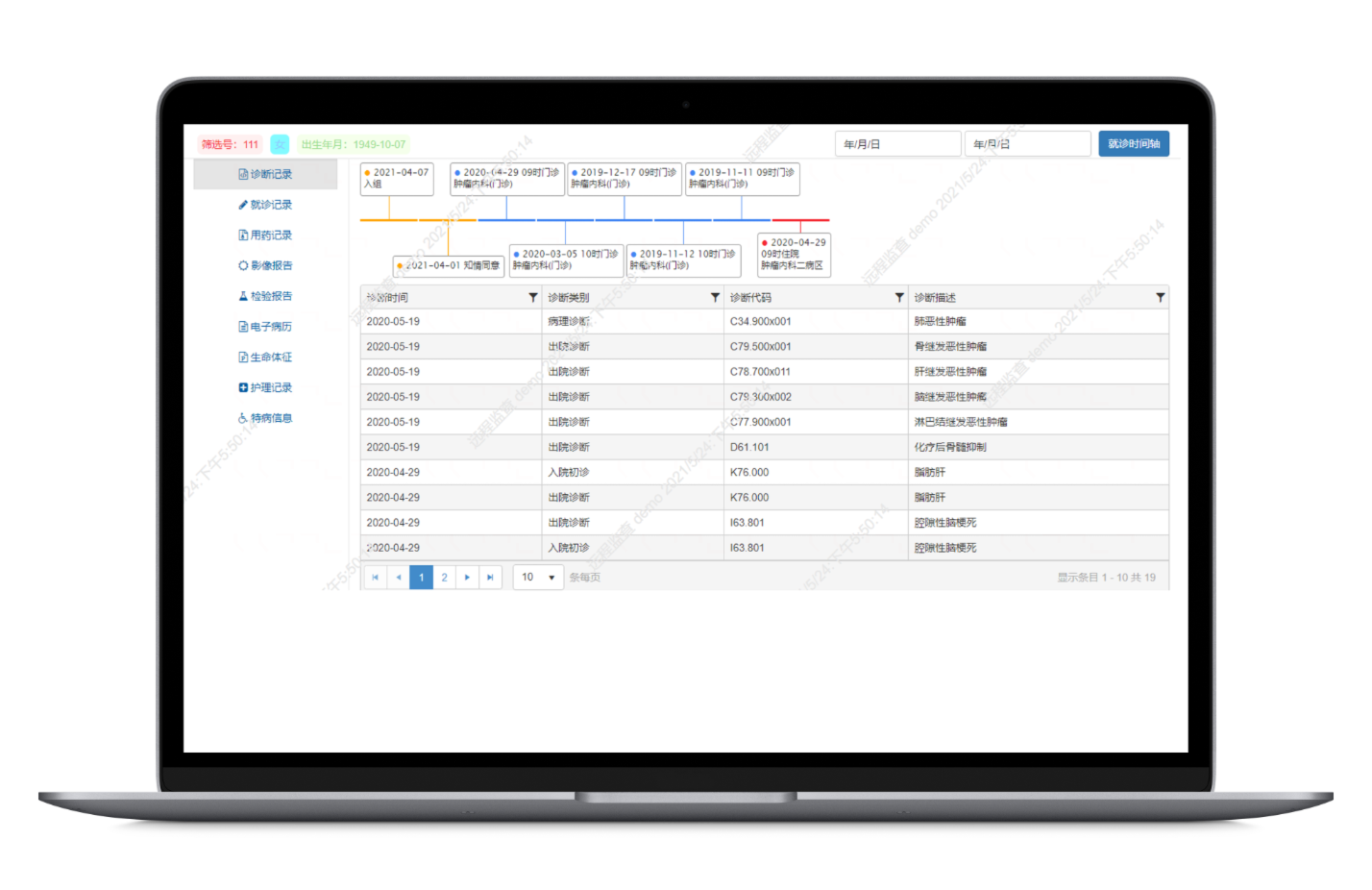
Using cohort design rules, map and transform data from hospital systems, EDC, and patient follow-up records to create a specialized research database tailored to specific study needs
Speed up recruitment by intelligently match potential subjects
Use text mining to efficiently sort through recruitment datasets
Integrate hospital-wide patient data to broaden the pool of potential subjects
Automatically capture research data using CDISC standards for clinical data conversion and transfer via ODM to EDC
Leverage natural language processing for large volumes of unstructured data, such as electronic medical records and test reports
Ensure full traceability of the data flow process
Leverage the GCP-X platform for real-time remote monitoring with a 360° view of patient clinical trials
Automatic logic checks and multi-layered intelligent quality control boost institutional QC efficiency
A robust security system with data de-identification, audit trails, and automatic watermarking ensures data and patient privacy
Local deployment prevents data leakage, keeping all data within the hospital

Scan to Follow Us on LinkedIn
