
Streamlines document creation, approval, archiving, and QC for accurate, compliant records and seamless audits.
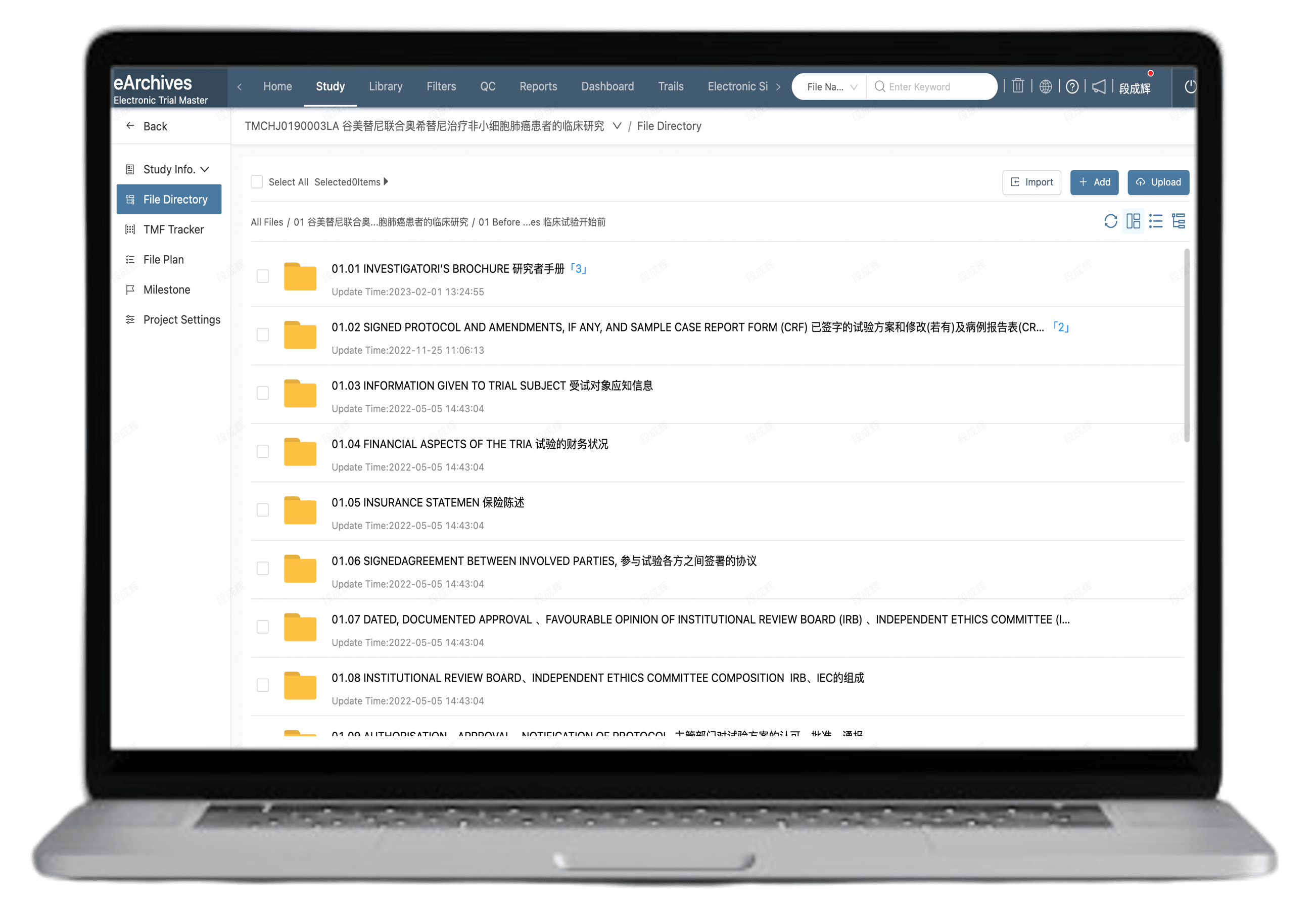
Includes standard TMF templates, SOP templates, and planning document librarie
Industry-standard templates: DIA eTMF RM V3.2, ICH & NMPA-GCP Essential Docs
One-click application for fast TMF Index creation with dynamic adjustments
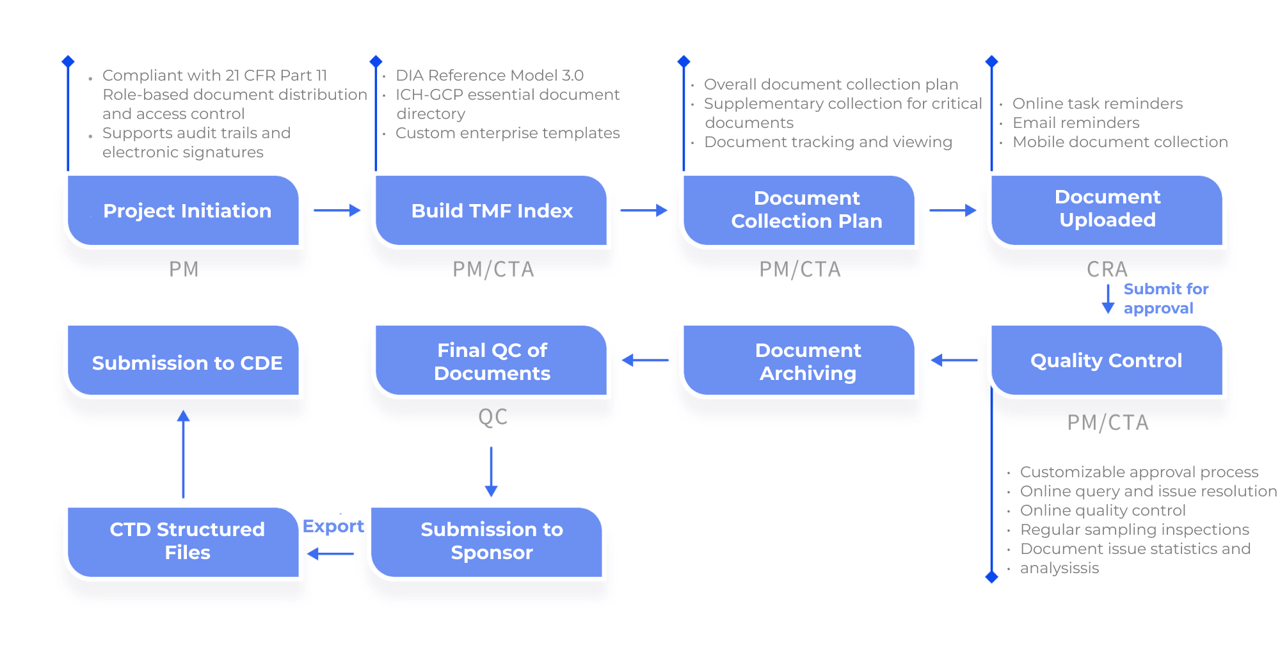
Document tracking, timely task assignment and process monitoring
Automated reminders for document collection to prevent omissions and delays
Mobile app configuration for quick and easy file uploads via photo scanning
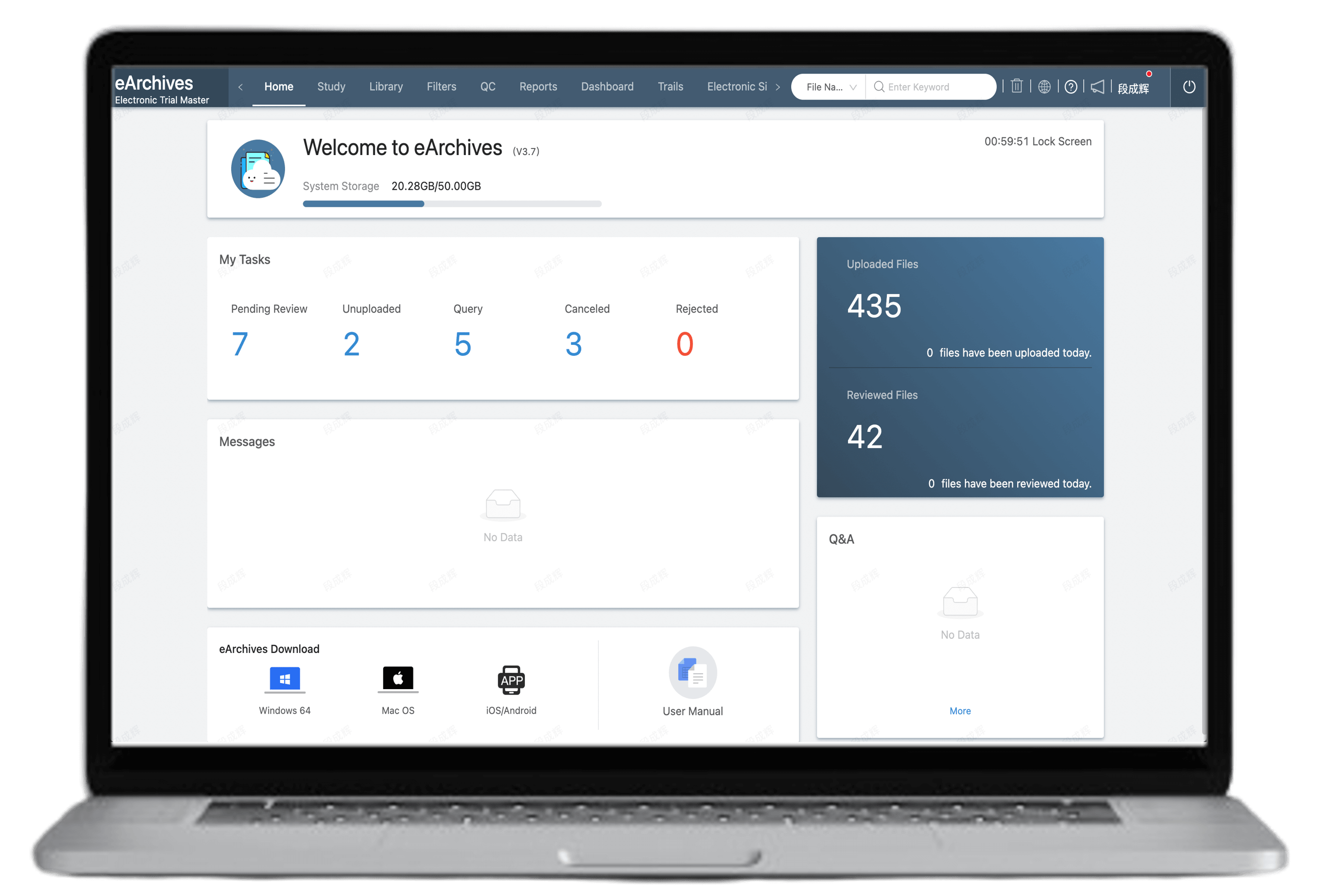
Customizable approval workflows to fit different enterprise’s SOPs
Multi-dimensional reports with real-time visual tracking of document status
Role-based access control ensures document security, with full traceability
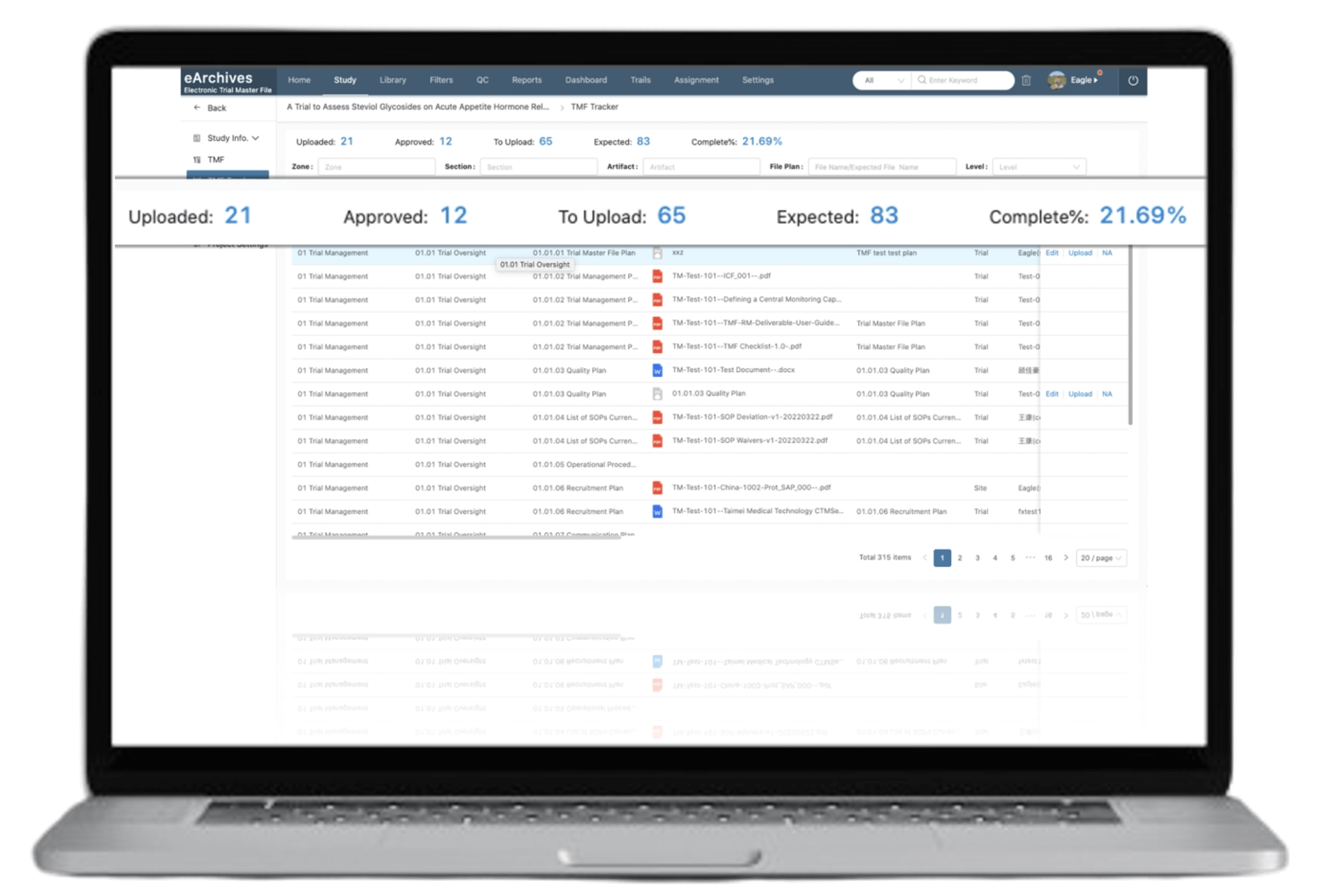
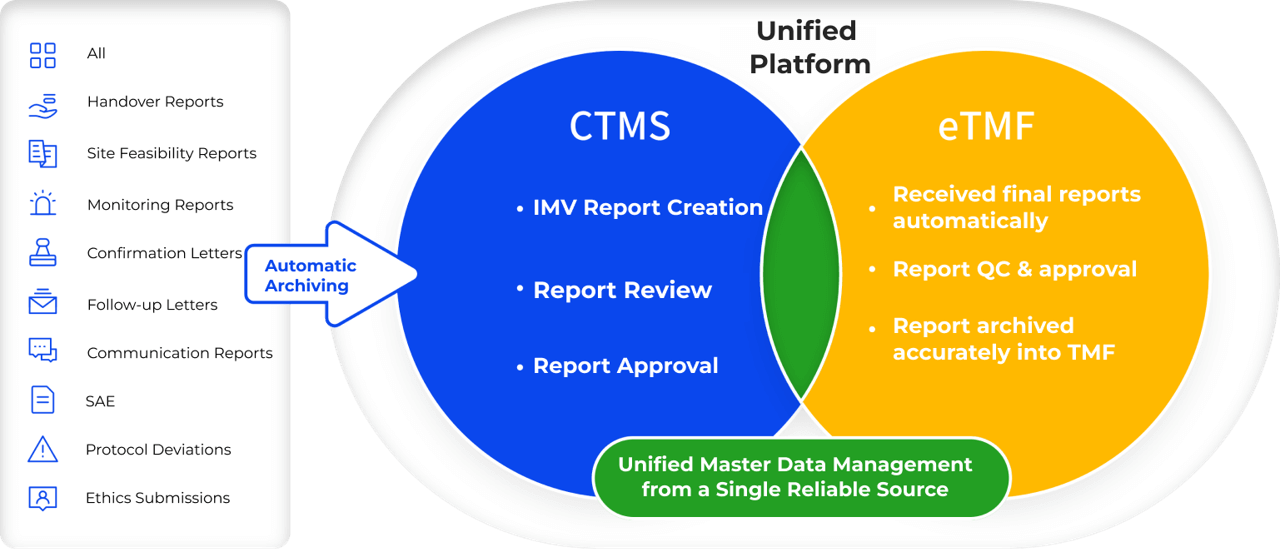
Single source: one platform for CTMS and eTMF documents, eliminating duplicate copies across locations
Reduces CRA workload: reports are automatically filed in the eTMF project folder after being processed in CTMS

Scan to Follow Us on LinkedIn

Scan to Follow Us on WeChat